
Infectious diseases were the leading causes of death on the first Labor Day, just ahead of a major epidemiological shift that brought both vaccines to fight these deadly ailments but also the rise of cigarette smoking.
Mary Caffrey is the Executive Editor for The American Journal of Managed Care® (AJMC®). She joined AJMC® in 2013 and is the primary staff editor for Evidence-Based Oncology, the multistakeholder publication that reaches 22,000+ oncology providers, policy makers and formulary decision makers. She is also part of the team that oversees speaker recruitment and panel preparations for AJMC®'s premier annual oncology meeting, Patient-Centered Oncology Care®. For more than a decade, Mary has covered ASCO, ASH, ACC and other leading scientific meetings for AJMC readers.
Mary has a BA in communications and philosophy from Loyola University New Orleans. You can connect with Mary on LinkedIn.

Infectious diseases were the leading causes of death on the first Labor Day, just ahead of a major epidemiological shift that brought both vaccines to fight these deadly ailments but also the rise of cigarette smoking.
In this review, authors explain the supply chain issues of getting a life-saving treatment "vein to vein."

United Urology Group, with 4 practices in Maryland, Colorado, Arizona, and Tennessee, has agreed to be acquired by OneOncology, which last year was acquired by TPG and AmeriSourceBergen in a $2.1 billion transaction.


A review of the upcoming schedule of the Institute for Value-Based Medicine.



The immunotherapy combination was initially more costly but resulted in a lower cost of care over a 2-year period, investigators found.

A session on the final day of the 2024 Congress of the American Society of Preventive Cardiology explored the connections between autoimmune disease and cardiac events.
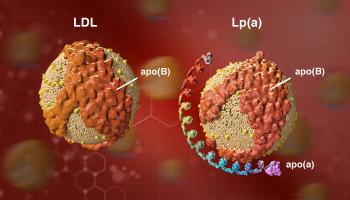
A debate on whether testing Lp(a) levels is actionable in primary prevention today offered lively discussion at the 2024 Congress of the American Society for Preventive Cardiology, being held in Salt Lake City, Utah.
A joint session with the Society for Cardiovascular Computed Tomography (SCCT) took place on the first day of the American Society for Preventive Cardiology 2024 Congress in Salt Lake City, Utah.








New Jersey Governor Phil Murphy joined BeiGene CEO John Oyler and about 300 guests to open the facility, located in Hopewell, New Jersey. A version of this article appeared in the August issue of Evidence-Based Oncology.

We recently spoke with Tycel Phillips, MD, associate professor, Division of Lymphoma, Department of Hematology & Hematopoietic Cell Transplantation, City of Hope, about his team’s interim analysis of their dose-escalation study of glofitamab against relapsed/refractory B-cell non-Hodgkin lymphoma.

Matthew Zachary, the keynote speaker for Patient-Centered Oncology Care 2024, recalls what the treatment experience was like when he was diagnosed with a rare pediatric brain cancer in 1996. Zachary is now a patient advocate.
A long-awaited report from the Federal Trade Commission (FTC) finds that vertical integration and consolidation have worked against consumers and independent pharmacies.

Researchers from the University of Manchester sought to understand whether the season of administration of immunotherapy affected outcomes.


A study finds limited changes in hospice utilization, highlighting challenges in real-world implementation.

Interview on Ontada research presented at ISPOR 2024.

Interview on Ontada research presented at ISPOR 2024.

Interview on Ontada research presented at ISPOR 2024.

Interview on Ontada research presented at ISPOR 2024.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
