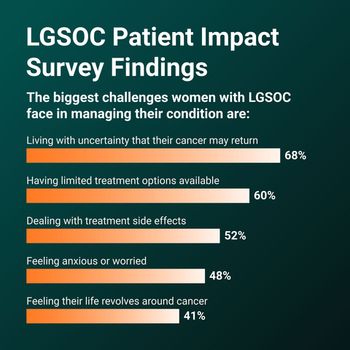
Desmoid tumors are noncancerous growths that appear in connective tissue, most often on the arms, legs, and abdomen. Aggressive tumors must be treated with surgery, radiation, or chemotherapy to keep them from growing into nearby organs, which can make them life threatening. They can cause pain and disfigurement, and they can reduce functioning and quality of life.