
The study was launched the same month as the first US approval of a sodium-glucose co-transporter 2 inhibitor, and the failure to include a drug from this class was viewed by a commentator as a weakness of GRADE.
Mary Caffrey is the Executive Editor for The American Journal of Managed Care® (AJMC®). She joined AJMC® in 2013 and is the primary staff editor for Evidence-Based Oncology, the multistakeholder publication that reaches 22,000+ oncology providers, policy makers and formulary decision makers. She is also part of the team that oversees speaker recruitment and panel preparations for AJMC®'s premier annual oncology meeting, Patient-Centered Oncology Care®. For more than a decade, Mary has covered ASCO, ASH, ACC and other leading scientific meetings for AJMC readers.
Mary has a BA in communications and philosophy from Loyola University New Orleans. You can connect with Mary on LinkedIn.

The study was launched the same month as the first US approval of a sodium-glucose co-transporter 2 inhibitor, and the failure to include a drug from this class was viewed by a commentator as a weakness of GRADE.
Sanofi gave up rights to develop the glucagon-like peptide 1 receptor agonist last year after a change in strategy.

How therapeutic advances can address all 3 conditions was the topic of a symposium Friday during the 81st Scientific Sessions of the American Diabetes Association (ADA), “The Intersection of Diabetes, Heart Failure and Kidney Disease: Challenges and New Insights.”

The once-weekly therapy is also being studied in nonalcoholic steatohepatitis and heart failure with preserved ejection fraction and obesity.

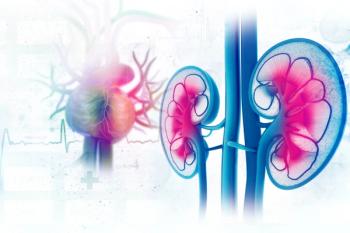
Updates reflect the increased intersection among diabetes, cardiovascular, and kidney disease care among certain drug classes, especially sodium glucose co-transporter 2 (SGLT2) inhibitors.

The American Diabetes Association's chief scientific and medical officer said the results are important to families at risk of type 1 diabetes.

Robert A. Gabbay, MD, PhD, the chief science and medical officer at the American Diabetes Association (ADA) offers insights on trial results and sessions to be presented at this year's ADA Scientific Sessions, taking place virtually June 25-29, 2021.

Robert A. Gabbay, MD, PhD, the chief science and medical officer at the American Diabetes Association (ADA), previews sessions to be presented at the ADA's 81st Scientific Sessions.



The study's lead author said researchers have searched for a generation for an effective therapy to give to high-risk patients with renal cell carcinoma after initial surgery for the tumor.

Also, phase 2 results for atezolizumab presented during the 2021 Annual Meeting of the American Society of Clinical Oncology could help patients in same setting who need another option.

Biomarker testing and remote patient monitoring are just 2 areas of research presented during the annual meeting of the American Society of Clinical Oncology (ASCO).

Equality in cancer care is not sufficient, said Lori Pierce, MD, FASTRO, FASCO, a radiation oncologist from the University of Michigan and president of the American Society of Clinical Oncology (ASCO). Equity—which means that patients have similar outcomes, regardless of circumstance—is harder to achieve.

During the first day of the 2021 Annual Meeting of the American Society of Clinical Oncology, a discussion on disparities in women's cancer care highlighted challenges in the United States and overseas.

ASCO officials characterized the results as practice changing and said they highlighted the need for genetic testing in patients who receive a diagnosis of high-risk breast cancer.

Humana's Bold Goal program allowed the health care giant to test new delivery models that it used to address loneliness and food insecurity during COVID-19.

Martin J. Edelman, MD, chair of the Department of Hematology/Oncology and associate director for Translational Research at the Fox Chase Cancer Center in Philadelphia, Pennsylvania, discusses the the nuances of rescinded indications in small-cell lung cancer.

Because Medicaid has always been a shared program between federal and state governments, there has been wide variation in income limits. Today, 38 states and the District of Columbia have Medicaid expansion.

When drug developers were forced to conduct cardiovascular outcomes trials for SGLT2 inhibitors, they found a surprise: the drugs created for type 2 diabetes (T2D) had strong benefits for heart failure. And the same has proven true in sotagliflozin.

The study presented during the American College of Cardiology's 70th Scientific Session called for one group of hospitals to receive special audits and guidance aimed at improving care of patients with heart failure.

Sessions, posters, and late-breaking trials at the American College of Cardiology’s 70th Scientific Session offer updates on vericiguat, SGLT2 inhibitors, sacubitril/valsartan, and heart failure therapies still in the pipeline.
Frank Martin, PhD, director of research at JDRF, discusses the organization's efforts to educate regulators about the disease.

Two top cardiologists debated evidence involving one clinical trial for omega-3 fatty acids, with implications for another, the REDUCE-IT study for icosapent ethyl (Vascepa).
The Dapagliflozin in Respiratory Failure in Patients With COVID-19 trial is the first phase 3 study to examine whether this SGLT2 inhibitor, which has proven effective for multiple chronic conditions, might be similarly useful in an acute setting.

The investigational drug for obstructive hypertrophic cardiomyopathy is in front of FDA, with an approval target date of January 28, 2022.

Results presented at the American College of Cardiology's 70th Scientific Session show most patients prefer a smaller dose and are more likely to stick with it.

Fourteen months after the American College of Cardiology (ACC) switched its 17,000-person meeting to a virtual format on short notice, the meeting will be online May 15-17 for the second year. The 70th Scientific Session will feature 25 late-breaking clinical trials, emphasizing treatment of heart failure and the right aspirin dose for prevention of secondary cardiovascular disease.

Frank Martin, PhD, director of research at JDRF, shares feedback from patients on JDRF's T1Detect screening program.
Both studies were featured during the American College of Cardiology's annual briefing on results for consumers. They will be presented during the 70th Scientific Session, which is set for May 15-17, 2021.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
