Oncology
Latest News
Latest Videos

More News
A phase 2b clinical trial is producing promising results for a personalized cancer vaccine used in patients with stage III and IV resected melanoma.


Financial toxicity, ACO contracts, removing barriers to care.

Selected abstracts from the 2019 Annual Meeting of the American Society of Clinical Oncology.

Research on biosimilars presented at the 2019 Annual Meeting of the American Society of Clinical Oncology.

From the Oncology Care Model to the possibilities of cannabis, policy issues drew big crowds at the 2019 Annual Meeting of the American Society of Clinical Oncology.

Cytokine release syndrome represents a major concern, and source of costs, associated with the life-saving gene therapy.

Real-world evidence is being embraced by FDA as a way to expand the review of data beyond the trial population, which may not be representative of the patients seen in clinics on a daily basis.

Clinical findings presented at the 2019 Annual Meeting of the American Society of Clinical Oncology in Chicago, Illinois.
The combination of nivolumab (Opdivo) and ipilimumab (Yervoy) demonstrated significant clinical benefit in patients with solid pediatric tumors that progressed in adulthood, a patient population with few treatment options, according to study findings presented at the 2019 American Society of Clinical Oncology Annual Meeting in Chicago, Illinois.

Although smoking cigarettes is thought of as being the most prevalent—yet preventable—cancer risk, new data suggests people who are obese now outnumber people who smoke, with excess weight causing more cases of certain cancers than smoking in the United Kingdom.
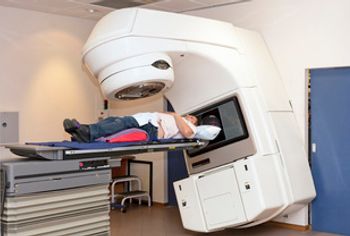
CMS has proposed the mandatory Radiation Oncology Model, which would cover radiation therapy spanning a 90-day episode.

Poor interpretation of imaging is the top reason for oncology malpractice lawsuits.

Time to diagnosis, the interval that passes from first symptom presentation until diagnosis, varies by cancer type among children, adolescents, and young adults and may be affected by clinical and sociodemogrpahic factors.

New innovations in cancer therapies have people excited to leave behind the treatments of old, but it might not be time yet to throw away chemotherapy, said Bruce Feinberg, DO, vice president and chief medical officer of Cardinal Health Specialty Solutions.
Last week, a panel of diverse stakeholders took the stage at the National Comprehensive Cancer Network Policy Summit in Washington, DC, to discuss shifting regulatory action from the federal to the state level, and the possible implications for patient access to high-quality cancer care.
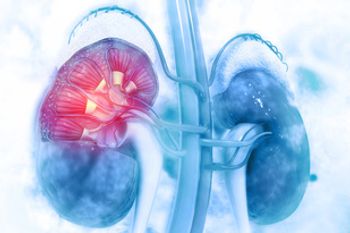
Evidence has shown that nivolumab immunotherapy could effectively treat metastatic renal cell carcinoma (RCC) in settings where resources are constrained.

Here are 5 interesting findings from the June 2019 issue of AJMC®.

If chemotherapy is still going to be used for another decade, there needs to be continued research into it and how to improve quality of life for patients treated with it, said Bruce Feinberg, DO, vice president and chief medical officer of Cardinal Health Specialty Solutions.

A report released Thursday from the National Academies of Sciences, Engineering, and Medicine said the United States needs a US National Cancer Control Plan that uses a system approach to reshape cancer control efforts, targeting everything from prevention, machine learning, palliative care, assessing value, rooting out financial conflicts of interest, and more.

OBR hosted a webinar that featured a discussion about how oncology practices can leverage artificial intelligence (AI) to improve cancer care.

According to the researchers, these rates are nearly double that seen in the general population, which suggests the presence of unmet needs among the survivorship community.



AJMC®TV interviews let you catch up on what’s new and important about changes in healthcare, with insights from key decision makers—from the clinician, to the health plan leader, to the regulator. When every minute in your day matters, AJMC®TV interviews keep you informed. Access the video clips at ajmc.com/interviews.