Immuno-Oncology
Latest News

Latest Videos

CME Content
More News

Data suggest that the technology may be an option for patients who are ineligible for autologous stem cell transplantation (ASCT) based on certain patient characteristics, prior treatments, stem cell availability, or tumor chemosensitivity.

Despite the obstacles and risks, the review authors conclude that universal chimeric antigen receptor (CAR) T-cell therapy has the potential to play a key role and mitigate some of the limitations associated with autologous CAR T-cell therapy in cancer treatment.
The review concludes that CD20-targeted chimeric antigen receptor (CAR) T-cell therapy is an option worth exploring, despite potential safety concerns that warrant more extensive research.
Renal outcomes and chimeric antigen receptor (CAR) T-cell therapy efficacy were unaffected by baseline renal status in a cohort of patients with diffuse large B cell lymphoma, but acute kidney injury during treatment was associated with worse clinical outcomes.
The review highlights current strategies for myeloid cell targeting and novel agents targeting myeloid cells for cancer treatment.
A significant portion of patients who receive chimeric antigen receptor (CAR) T-cell therapy experience immune effector cell–associated neurotoxicity syndrome, and this recent study suggests neurofilament light chain protein levels may hold promise as a biomarker to identify at-risk patients.

The review of 5 phase 3 randomized controlled trials found that overall, the combination treatments improved survival outcomes while eliciting more, but manageable, side effects.

Wayne Winegarden, PhD, of the Pacific Research Institute, explains the harm that white bagging policies cause health systems and how replacing them with new measures to boost market competition could help patients.
Researchers studied data from 5 phase 1 studies that took place between 2012 and 2021, comprising 139 infusions among patients with B-cell acute lymphoblastic leukemia who received chimeric antigen receptor (CAR) T-cell treatment.

Despite advances made against the disease, researchers note that breast cancer still remains a top cause of cancer-related mortality in women around the world.

With 40% of patients with diffuse large B-cell lymphoma experiencing relapsed or refractory disease (rrDLBCL), researchers highlighted the promise of chimeric antigen receptor (CAR) T-cell therapy for these patients.
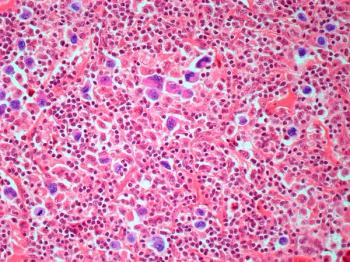
Both the ZUMA-7 trial for axicabtagene ciloleucel—approved for second-line treatment earlier this year—and the TRANSFORM trial for lisocabtagene maraleucel showed significant improvement over standard of care for large B cell lymphoma (LBCL), while the BELINDA trial for tisagenlecleucel did not.

Data presented at the American Society of Clinical Oncology annual meeting found non-White patients are less likely to receive immunotherapy for head and neck cancer, said Amila Patel, PharmD, chief clinical officer, Navigating Cancer.

Overall, infections occurred in 18.42% of cases and typically occurred within the first month, significantly dropping from then on.




ASCO Spotlight With Kim A. Reiss, MD, on CAR Macrophages and Other Developments in Pancreatic Cancer
Kim A. Reiss, MD, assistant program director, assistant professor of medicine, Hospital of the University of Pennsylvania, Philadelphia, discusses 2 abstracts presented during ASCO.


Abstracts presented at this year's American Society of Clinical Oncology Annual meeting detailed the financial hardships many patients with cancer face, including those related to high-deductible health plans and clinical trial participation.

Zahra Mahmoudjafari, PharmD, BCOP, clinical pharmacy manager at the University of Kansas Health System, explains some of the strategies that payers can use to improve cost management and increase patient access related to chimeric antigen receptor T-cell (CAR T) therapies.

Zahra Mahmoudjafari, PharmD, BCOP, clinical pharmacy manager at the University of Kansas Health System, catalogues the chimeric antigen receptor T-cell (CAR T) therapies that are coming down the pipeline.

Miruna Sasu, PhD, MBA, became president and CEO of COTA Healthcare in March 2022, not quite a year after joining the company as its chief strategy officer. As the importance of RWD grows, Sasu is ready to take its use in precision oncology to new levels. She spoke with Evidence-Based Oncology™ (EBO) about her new role.

Major adverse cardiac events are rare but serious in patients treated with immune checkpoint inhibitors (ICIs), and increased awareness is crucial to identify and treat these conditions in a timely manner.