
Working from findings in the landmark TAILORx study, reseachers estimated that Oncotype DX-guided treatment could reduce the total first-year breast cancer care by nearly $50 million across the United States.


Working from findings in the landmark TAILORx study, reseachers estimated that Oncotype DX-guided treatment could reduce the total first-year breast cancer care by nearly $50 million across the United States.

Howard A. "Skip" Burris, III, MD, FACP, FASCO, president, clinical operations, and chief medical officer of Sarah Cannon Research Institute, discusses differentiating which patients will benefit from next-generation sequencing (NGS) and the role of NGS testing for matching patients to clinical trials.
It's estimated that by 2021, 100 million people will have used a direct-to-consumer (DTC) genetic test. As these tests continue to gain popularity, there is a need for educating consumers on their DTC testing results and validating these results with confirmatory testing in a medical-grade laboratory.
Circulating tumor DNA (ctDNA) can be analyzed in patients with cancer to detect minimal residual disease (MRD). Two abstracts, presented at the American Association for Cancer Research Annual Meeting 2019, evaluated ctDNA analysis and ways of tracking MRD.
The scientists used machine learning to test the algorithm and believe it could double the number of women who would be treated with poly (ADP-ribose) polymerase (PARP) inhibitors for breast cancer.

Through partnerships, CancerIQ tries to reduce barriers for patients to use genetic services to detect cancer early, said Feyi Olopade Ayodele, MBA, chief executive officer at CancerIQ.

The treatment of lung cancer with personalized medicine has come a long way in recent decades, but still more achievements remain to be seen, said Bruce Johnson, MD, FASCO, immediate past president of the American Society of Clinical Oncology, during his keynote speech opening the Business of Oncology Summit hosted by the Florida Society of Clinical Oncology in Kissimmee, Florida.
The filing comes as recent upgrades to NCCN guidelines expand the role of testing in the treatment of ovarian cancer.
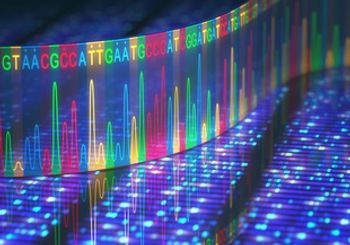
Genetic alternations in colorectal cancer (CRC) are linked to different survival and treatment outcomes, according to a study that used next-generation sequencing (NGS) of tumor DNA. The study was published in Journal of Clinical Oncology.

Healthcare experts may agree the shift from volume to value is well under way, but the definition of value has many answers, according to pharmaceutical company representatives discussing the issue at the 2019 Community Oncology Conference in Orlando, Florida.

Howard A. "Skip" Burris, III, MD, FACP, FASCO, president, clinical operations, and chief medical officer of Sarah Cannon Research Institute, discusses what's needed from a policy perspective to ensure access to next-generation sequencing (NGS) tests.

As novel therapies drive up the cost of drugs, it is becoming increasingly difficult for community oncologists to keep costs below value-based care program targets, according to a new survey from Integra Connect.
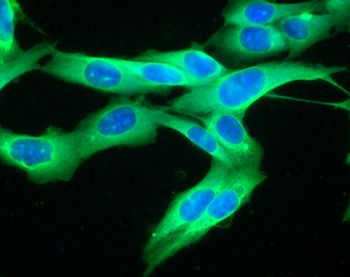
Recent work looking at the response to immune checkpoint blockade (ICB) therapy given before surgery for advanced melanoma found increased infiltration of B cells, a type of white blood cell, in patients who responded to therapy compared with those who did not.

A lack of diversity in cell lines used for laboratory studies means underrepresented populations and minorities might not benefit from precision medicines as quickly as people from European ancestry.

Biomarker testing will start to evolve to look more at combination therapies, said Jarushka Naidoo, MB BCh, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins.

A presentation Saturday at the National Comprehensive Cancer Network Annual Conference in Orlando, Florida, outlined several key updates for treatment in ovarian cancer based on new studies and approvals for poly (ADP-ribose) polymerase (PARP) inhibitors and bevacizumab.

Germline testing at diagnosis, along with tumor testing, have the potential to identify candidates for investigational poly (ADP-ribose) polymerase (PARP) inhibitors, and updated guidelines call for their expanded use.

CancerIQ is reducing barriers that make it difficult to utilize genetic testing to predict or pre-empt a patient’s cancer diagnosis, explained Feyi Olopade Ayodele, MBA, chief executive officer at CancerIQ.

Multigene testing provides a lot of information that providers have to be familiar with in order to adequately explain it to their patients, said John H. Ward, MD, Huntsman Cancer Institute at the University of Utah.
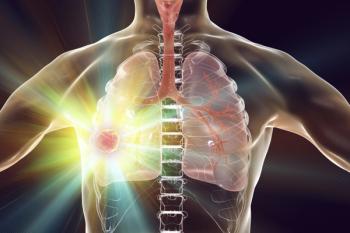
Guideline updates discussed Thursday at the 2019 National Comprehensive Cancer Network Annual Conference reflect recent practice-changing clinical trials involving pembrolizumab and chemotherapy.

Patients with high-risk breast cancer who receive a 21-gene assay genomic test may be able to avoid chemotherapy and ultimately save a significant amount of money.
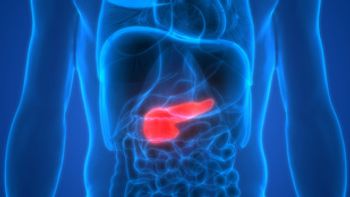
Finding a less invasive way of determing if pancreatic cysts are likely to become cancerous would be good for patients and less taxing on the healthcare system.
Minimal residual disease (MRD) testing is the future of diagnostics and monitoring for cancer, according to some experts. Not only can MRD determine the depth of response to a treatment, but it can also be used to determine treatment strategies.

Not having enough tissue is often a problem in precision medicine. The senior author of a new study said a liquid biopsy could become a new standard.
Two abstracts presented at the Transplantation and Cellular Therapy Meetings analyzed the detection of minimal residual disease during and after hematopoietic stem cell transplantation.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
