
A recently published study amassed real-world evidence from patients with chronic obstructive pulmonary disease (COPD), concluding that “there has been a closing of the gap in COPD prevalence among both genders.”

A recently published study amassed real-world evidence from patients with chronic obstructive pulmonary disease (COPD), concluding that “there has been a closing of the gap in COPD prevalence among both genders.”

The drug, HU-308, is aimed at treating levodopa-induced dyskinesia (LID). The animal study showed that it was as effective as amantadine, the only available treatment for dyskinesias; combining HU-308 with amantadine was also more effective than either drug used alone.
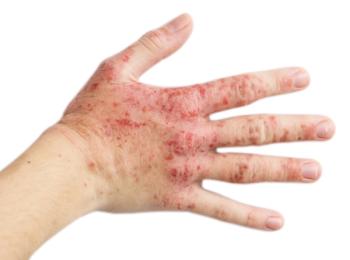
Current categories of classifying psoriasis, such as mild, moderate, or severe, do not consider past treatments or involvement of special areas, leading to undertreatment, a report said.

This week, top managed care news included a federal appeals court overturning the individual mandate; the Trump administration unveiling a plan to import low-cost drugs; FDA approving a popular fish oil pill to prevent heart attacks and strokes.

A new analysis says allowing beneficiaries in Medicare Part D to benefit from drug manufacturer rebates shows that they could save $29 billion. But if another proposal is chosen, they would save less, and taxpayer costs would rise.

Novartis hopes to distribute Zolgensma, its pricey spinal muscular atrophy drug, via lottery; Merck receives FDA approval for its Ebola vaccine, Ervebo; a new law in California hopes to resurrect compassionate use cannabis programs.

Coverage of our peer-reviewed research and news reporting in the healthcare and mainstream press.

The federal goverment proposed to allow states to import drugs from Canada, but many obstacles lie before its passing; FDA targeting e-cigs that have been linked to teen addiction with no attributable benefit among smoking cessation; inclusions and exclusions in the federal spending package set to be passed by Congress this week

A new sponsor will award a Massachusetts-based organization up to $25,000.

Findings of a review analyzing the association between cigarette smoking and multiple sclerosis (MS) highlight numerous detrimental effects the habit has on those with the disease. The review, published in the Journal of the America Medical Association (JAMA) Neurology, collected data from studies published between 1965 and 2018.
Hypertrophic cardiomyopathy (HCM) is an inherited condition in which mutations in genes that encode the sarcomere proteins in the heart cause an abnormal thickening of that muscle, with no known cure. In black patients, HCM is usually diagnosed at a younger age and accompanied by a greater burden of symptomatic heart failure. These patients, however, are not well represented in surveys of the condition, which tend to focus on white patients.
On behalf of the Hellenic Headache Society, a group of Greek researchers reported a consensus on the diagnosis and treatment of adult migraines in The Journal of Headache and Pain on December 13.

The National Business Group on Health released its list of healthcare trends to watch in 2020, highlighted by growing employer initiatives on high quality, high value healthcare, and an increased focus on mental health issues.

A federal appeals court today struck down the individual mandate—the heart of the Affordable Care Act (ACA) that requires everyone to have health coverage and lays the groundwork for a risk pool that is more balanced between the sick and the healthy, the young and the old.

Sleep fragmentation, which is defined by low sleep efficiency, was linked with higher odds of migraine onset on day 1, according to study findings published this week.

Annual results from a national survey about drug and alcohol use show more teens are vaping marijuana; HHS is trying to increase rates of organ donation; CVS is accused of fraudulent billing practices.
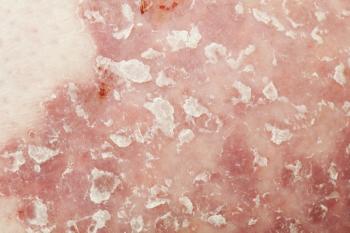
The investigational therapy, an interleukin (IL)-17A and IL-17F inhibitor, is the third phase 3 study of bimekizumab to report positive results since October, following BE VIVID and BE READY, which evaluated the safety and efficacy of the drug.

Through a Notice of Proposed Rulemaking and a draft guidance for industry, the FDA will seek request for comment on 2 pathways. One would allow states to submit proposals to the FDA to allow the importation of small molecule brand-name medicines sold at retail pharmacies. The other is a draft guidance for industry, which would let manufacturers import the same versions of FDA-approved drugs they now sell in foreign countries.

Narcolepsy management should be a combination of behavioral and pharmacologic strategies that are tailored to the patient with consideration of the impact of symptoms and how they may evolve, according to a recent review aiming to improve narcolepsy diagnosis and management.

Despite a 20% drop in mortality since 2009, colorectal cancer accounted for 9.8% (881,000) of deaths worldwide in 2018 and represents 10.2% of all cancer cases worldwide. It is No. 3 on the list of most prevalent cancers worldwide—1.8 million new cases in 2018—behind only lung cancer and breast cancer.
In cases of acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), there is a greater risk of symptomatic heart failure in the first year following initiation of anthracycline treatment.

Patients with breast cancer who have government insurance face a higher risk of death, according to a retrospective study presented at the 2019 San Antonio Breast Cancer Symposium on December 13.

Caregivers of patients with Parkinson disease (PD) experiencing symptom recurrence after a period of symptom control, known as OFF periods, reported that PD had a greater impact on their paid employment through variables such as loss of earnings, loss of opportunities, or ability to maintain employment compared with caregivers for patients without OFF periods.
Newly released statistics published in JAMA illustrate a sharp increase in unintentional US opioid deaths.

Open enrollment for ACA health plans is extended after glitches on Healthcare.gov; EPA watchdog found that the agency's health monitoring during Hurricane Harvey was lacking; Purdue Pharma splits ties with the lobbying organization PhRMA.

Disease burden is substantial for patients with myelofibrosis, even those with intermediate risk, and a not insubstantial percentage of patients have low or intermediate adherence during treatment, according to 2 abstracts from an Italian clinical trial presented at the 61st American Society of Hematology Annual Meeting & Exposition.
Researchers constructed a risk prediction model of hospitalizations due to heart failure (HHF) for patients with type 2 diabetes mellitus (T2DM), according to a Clinical Cardiology study.
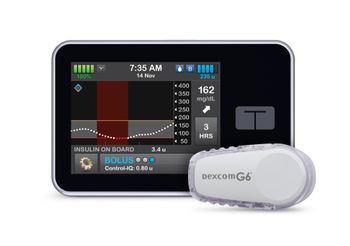
The company said that with the clearance, the FDA outlined standards for a new device category, the interoperable automated glycemic controller designation.
HIV-positive individuals face greater risks of kidney and liver diseases, cardiovascular events, osteoporosis, hepatitis C, and cancer. Clinical trials and research advances into the cause and development of the comorbid conditions are needed.

BMS and Gilead continue their fight over Yescarta patent infringement; Canadian researchers develop multidrug-resistant, bacteria-repelling plastic coating; Healthcare.gov floods with last-minute sign-ups on final day of enrollment for 2020 plans.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
