
Payers
Latest News
Latest Videos

Podcasts
CME Content
More News

Innovative financing and reimbursement models can improve access to cell and gene therapies, addressing cost barriers and improving patient outcomes, said Joe DePinto, MBA, of McKesson.

Pieter Sonneveld, MD, PhD, chair of the Erasmus MC Cancer Institute, discussed the findings of a study modeling long-term progression-free survival (PFS) in patients with multiple myeloma (MM) treated with a daratumumab quadruplet regimen.

To mark the 30th anniversary of The American Journal of Managed Care (AJMC), each issue in 2025 includes reflections from a thought leader on what has changed over the past 3 decades and what’s next for managed care. The May issue features a conversation with John Michael O’Brien, PharmD, MPH, a member of AJMC’s editorial board and the president and CEO of the National Pharmaceutical Council. This interview has been lightly edited for clarity.

Approximately 1 million Aetna members will need new coverage with the announcement that CVS will be leaving the Affordable Care Act (ACA) individual exchange business next year.

The results offer some of the first glimpses into real-world findings of the Janus kinase inhibitor in patients with myelofibrosis (MF) and anemia.

As the number of cell and gene therapies expands, it's increasingly important for long-term patient data, explained Fran Gregory, PharmD, MBA, vice president of emerging therapies at Cardinal Health.

As drug denials increase, experts discuss the importance of optimizing data to keep up with these changes and implementing artificial intelligence (AI) to reduce the burden on providers and ensure patient access to care and treatment.
Progression-free survival improvement and drug costs make zanubrutinib a more cost-effective option in relapsed or refractory chronic lymphocytic leukemia (CLL), new research suggests.

None of the eteplirsen-treated patients reached a left ventricular ejection fraction below 50% compared with 22.1% of patients in the control group.

The California-based entities plan to offer new Medicare Advantage (MA) products in select counties by this fall.
Investigators highlighted Raman spectroscopy, a noninvasive diagnostic technology that could lead to faster, earlier detection of skin cancer.
Researchers warned that to achieve such reductions, greater uptake of the medication among indicated patients is needed.

This study examined postdiagnosis breast cancer treatment outcomes for Medicare Advantage vs fee-for-service (FFS) Medicare in Ohio and found no significant differences overall but disparities for Black patients with FFS Medicare.

Prior authorizations create substantial administrative and financial burdens on physicians and patients and can disrupt the continuity of care.

A real-world analysis showed a link between continuous glucose monitor (CGM) distribution channel and outcomes for patients with diabetes.

The approval of revakinagene taroretcel (Encelto; Neurotech) addresses a significant unmet need for patients with macular telangiectasia type 2 (MacTel), clinical investigator Charles C. Wykoff, MD, PhD, Retinal Consultants of Texas, said.

Resmetirom offers a targeted approach to metabolic dysfunction–associated steatohepatitis (MASH) for use alongside lifestyle modifications.

Charles C. Wykoff, MD, PhD, Retinal Consultants of Texas, discusses data supporting the FDA approval of revakinagene taroretcel-lwey (Encelto; Neurotech Pharmaceuticals), the first and only therapy indicated for the treatment of macular telangiectasia type 2 (MacTel).

Tenacious efforts at every level, from the individual clinician to the hospital to the state to Congress, will be needed to make sure patients can access life-saving gene therapies for neuromuscular diseases.

Posters presented at the 2025 Muscular Dystrophy Association (MDA) Clinical & Scientific Conference show that therapeutic advances in treating spinal muscular atrophy (SMA) are not uniformly making it into the hands of patients who could benefit.

As health care costs continue to rise and the burden of chronic disease grows, data-driven insights will be essential in shaping the future of patient care, according to experts from Komodo Health and SmarterDx.

The study found that just 20% of trials submitted to both the FDA and European Medicines Agency had matching evidence.
An oncologist from Atrium Health outlines how saving time administering immunotherapy could have far-reaching benefits.
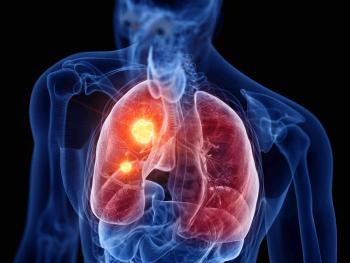
Artificial intelligence (AI) helps a Sarasota, Florida, health system catch lung nodules that appear on CT scans for patients treated for scores of conditions, allowing them to be referred for a possible lung cancer diagnosis.

Revakinagene taroretcel-lwey (Encelto; Neurotech), an allogeneic encapsulated cell-based gene therapy, is the first therapy to be approved for macular telangiectasia type 2.





















