Clinical
Latest News

Latest Videos

More News

The 2-day meeting of the American Cough Conference will cover new developments in the treatment of cough, as well as insights into the mechanisms and management of cough, said Peter Dicpinigaitis, MD, chair of the American Cough Conference.

Treating cough can be difficult. There are no FDA-approved therapies on the market in the United States, and companies with drugs in clinical trials have to contend with a large placebo effect.
If 2020 was dominated by the news of how COVID-19 spread across the globe, then 2021 has so far been focused on ending the pandemic through vaccine distribution.
Celltrion Healthcare’s high-concentration adalimumab biosimilar demonstrated comparable safety, efficacy, and immunogenicity profiles to the reference product for up to a year, providing further evidence supporting use of the product.

About 5.4% of patients with end-stage renal disease (ESRD) who begin renal replacement therapy as children go on to have vascular events, and 4.1% of those patients die as a result of vascular events, though the real incidence rates are likely higher, according to new research.

The FDA releases label changes for ozanimod to treat multiple sclerosis; the National Institute of Allergy and Infectious Diseases initiated a study on mixing COVID-19 vaccines; gender-affirming hormones may affect the cardiovascular system.
Researchers outlined novel approaches to prevent and manage cytokine release syndrome (CRS), which occurs frequently in patients receiving chimeric antigen receptor T-cell therapy.
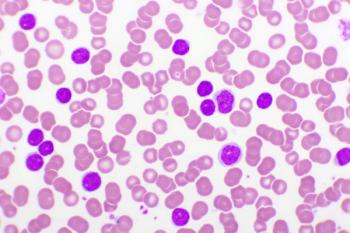
New studies evaluate the impact of age on overall survival in adolescents and young adults (AYAs) with chronic lymphocytic leukemia, as well as response rates of rituximab in 2 combination regimens for hairy-cell leukemia and follicular lymphoma.

A phase 3 trial explored which treatment option for pemphigus vulgaris, a rare skin disease, resulted in better outcomes after 52 weeks.

Martin J. Edelman, MD, chair of the Department of Hematology/Oncology and associate director for Translational Research at the Fox Chase Cancer Center in Philadelphia, Pennsylvania, discusses the the nuances of rescinded indications in small-cell lung cancer.

Recent coverage from Targeted Oncology® highlights how combination therapies are impacting the treatment spectrum for chronic lymphocytic leukemia (CLL).

A recent analysis of The Environmental Determinants of Diabetes in the Young study found maternal diet has no impact on type 1 diabetes and islet autoimmunity (IA) risk.

Some recent oncology news includes research into factors impacting the well-being of childhood cancer survivors; whether COVID-19 accommodations will last post-pandemic; and a possible new therapy for patients with polycythemia vera.
Birth control guidance provided to women taking rheumatoid arthritis therapies is lacking, and other rheumatology news reported across MJH Life Sciences™.

With a variety of treatments available for chronic lymphocytic leukemia (CLL), a disease that is extremely heterogeneous, clinicians are faced with a challenging task to choose the right treatment for a patient.

The latest coverage in the heart failure space from across MJH Life Sciences™.
After switching to 1 of 2 adalimumab biosimilars, patients with inflammatory bowel disease (IBD) experienced comparable clinical outcomes to the reference product after 6 months, according to a recent study.

Expanded coverage under a Medicare-Medicaid partnership to treat all prevalent cases of hepatitis C appears to be cost-effective by saving money and improving patient outcomes.

During the ATS 2021 International Conference, several studies shed new light on 2 asthma biologics.

Sessions, posters, and late-breaking trials at the American College of Cardiology’s 70th Scientific Session offer updates on vericiguat, SGLT2 inhibitors, sacubitril/valsartan, and heart failure therapies still in the pipeline.

Two top cardiologists debated evidence involving one clinical trial for omega-3 fatty acids, with implications for another, the REDUCE-IT study for icosapent ethyl (Vascepa).

Anthony Fauci, MD, told attendees at the ATS 2021 International Conference that the real-world evidence of vaccines against SARS-CoV-2, the virus that causes COVID-19, is even better than expected.
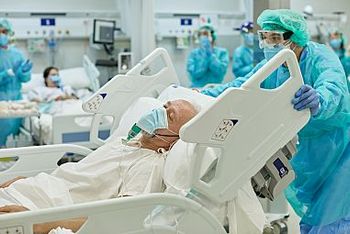
The Dapagliflozin in Respiratory Failure in Patients With COVID-19 trial is the first phase 3 study to examine whether this SGLT2 inhibitor, which has proven effective for multiple chronic conditions, might be similarly useful in an acute setting.

Hospitalized infants with the rare metabolic disorder maple syrup urine disease (MSUD), who are intolerant to oral or enteral administration of branched-chain amino acid-free formula, may benefit from an intravenous formulation.