Clinical
Latest News
Latest Videos

More News

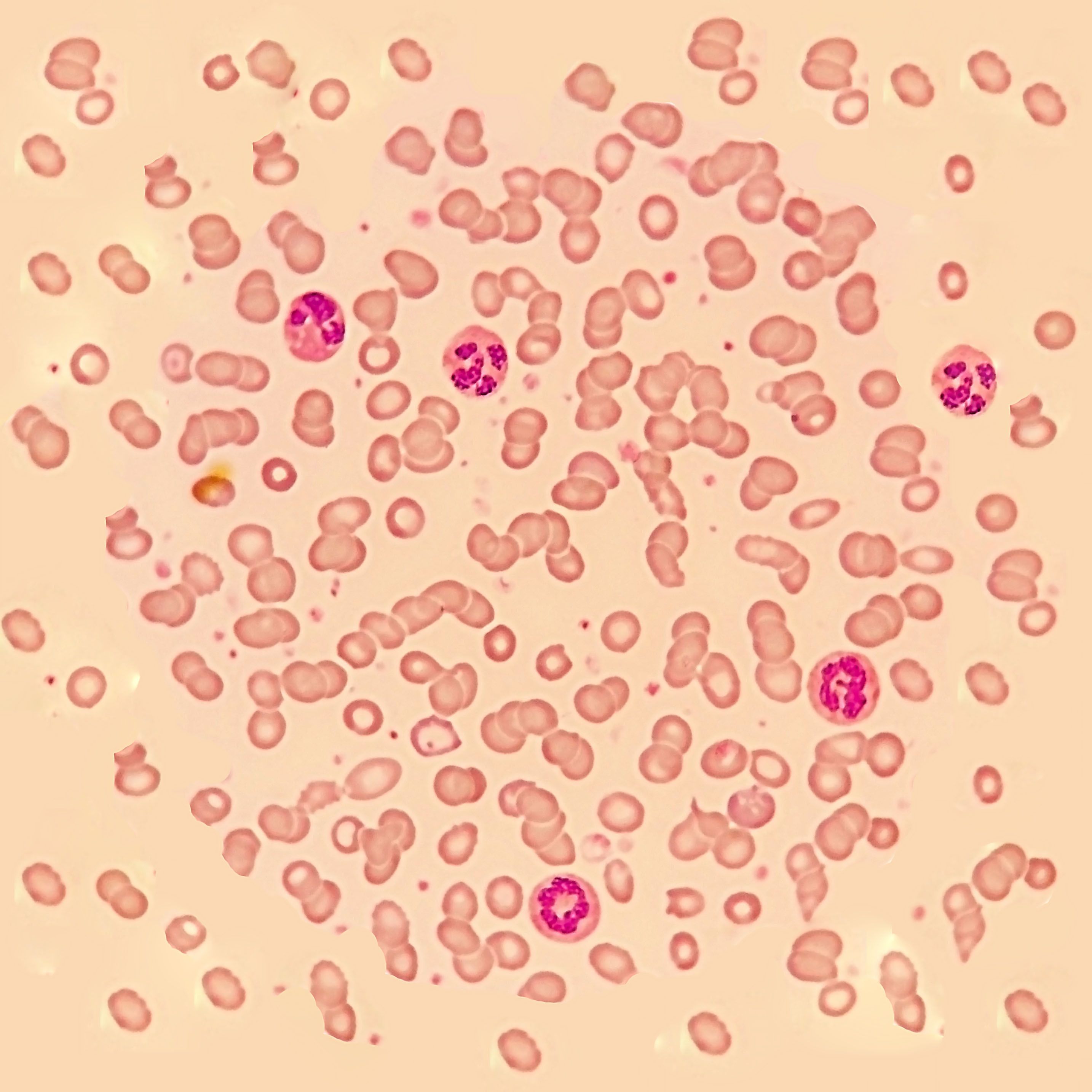
Multiple myeloma is the second most common blood cancer, affecting more than 130,000 US patients.
In patients with chronic inflammatory rheumatic and musculoskeletal diseases (RMD), the presence of metabolic syndrome (MetS) indicates an increased risk of cancer.

Study findings indicate that droplet digital polymerase chain reaction (ddPCR) may be more accurate than real-time quantitative PCR in measuring minimal residual disease (MRD) among pediatric patients with acute lymphoblastic leukemia (ALL), particularly in late follow-up time points.

Anemia, which occurs in over 50% of patients with end-stage kidney disease, should not be considered an obstacle to home dialysis, according to a new report.

Russell Langan, MD, chief of Surgical Oncology and Hepatopancreatobiliary Surgery at Saint Barnabas Medical Center, details the implementation of the center's Pancreatic Cyst Surveillance Program.

As more therapies continue to be approved, efforts will focus on toxicity prevention and mitigation strategies for associated unique and potentially life-threatening adverse reactions.

A recent subgroup analysis of the DAPA-HF study investigated possible clinical outcome differences between women and men following the addition of dapagliflozin to their treatment regimens.
Among patients with multiple sclerosis, there is a 16% greater risk of infection, including for lower respiratory and herpes virus infections, after administration of fingolimod.
Two therapeutic regimens used for the treatment of chronic obstructive pulmonary disease (COPD) are rarely being prescribed to patients without a COPD diagnosis, investigators concluded.

An ongoing multicenter study in Japan will evaluate reductions in pulmonary vascular resistance 8 months after initiating the combination therapy in pulmonary arterial hypertension.

President Biden outlines new vaccine goal; the United States will stop distributing Eli Lilly's bamlanivimab; rates of human papillomavirus (HPV) decrease among females.

After a 12-month follow-up of patients in the CHAMP-HF registry, Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OS) was shown to be more prognostically accurate compared with New York Heart Association functional class.
Rates of hepatocellular carcinoma were highest among persons living with HIV who have higher RNA levels, lower CD4 cell counts, or used injection drugs.
Among patients with atrial fibrillation (AFib) in the RATE-AF trial, the index-beat approach produced superior results on a potential diagnosis of heart failure vs consecutive-beat methods.
Patient data about emergency department use by this population is limited, the authors said.
A recent study found that a sizable portion of the commercially insured asthma population lacks disease control, regardless of asthma severity, signaling a needed shift in treatment strategies.

Improved understanding of the early mechanisms of chronic kidney disease (CKD) may be the next step in efforts to delay or reverse disease progression.

According to the researchers, few treatments have been able to reduce the thrombotic burden in patients with polycythemia vera (PV) and essential thrombocytopenia (ET), and progress on this front has been hindered by a lack of studies designed to assess a treatment’s impact on thrombotic events.
Spinal muscular atrophy treatment Zolgensma was found to be safe and effective for long-term use and use in presymptomatic patients, according to posters presented at the 2021 MDA Virtual Clinical & Scientific Conference.
Recent findings indicate that the symptoms of atopic dermatitis, hives, and rhinitis can all be linked to specific microbiota, implying that the gut-skin axis and gut-nose axis do exist.
There is a lack of standards for diagnosing and treating nail psoriasis, a situation needing attention because the condition is often a precursor to psoriatic arthritis.

Minimal residual disease (MRD) is being used more frequently in clinical trials and to identify the best treatment course for patients, but unknowns remain about the optimal use of MRD testing.

Patients with pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension had similar incidence of COVID-19, but the impact on clinical operations at the centers that treat these patients was substantial.

After Medicare Part D plans stopped covering a chronic obstructive pulmonary disease (COPD) therapy, patients had gaps in care and increased out-of-pocket costs, according to a recent study.

Field loss may not be the only visual deficit resulting from macular damage while resulting functional losses may impair facial recognition, according to new research published in JAMA Ophthalmology.