
Hematology
Latest News

Latest Videos
CME Content
More News

This new study evaluates the efficacy of monotherapies and venetoclax combination therapies in different T-cell acute lymphoblastic leukemia (ALL) cell lines.

The findings show most patients with low-risk myelodysplastic syndrome (MDS) will die before progression.

Researchers of a new study explore what areas of chronic lymphocytic leukemia (CLL) treatment and care are important to patients and identify key areas for improvement.

Peer and survivorship support are lacking for patients living with multiple myeloma, leading investigators to evaluate a group-focused multidisciplinary intervention that encompassed physical and mental activities.

In this interview from our coverage of the European Hematology Association 2024 Congress, we spoke with Brian Koffman, MDCM, DCFP, FCFP, DABFP, MSEd, executive vice president and chief medical officer of the CLL Society and himself a survivor of chronic lymphocytic leukemia (CLL), to discuss treatment advancements and the importance of addressing both unmet needs and patient treatment preferences.

A trio of experts discuss the challenges of diagnosing and managing paroxysmal nocturnal hemoglobinuria (PNH), a rare and life-threatening disorder.

Patients with myelodysplastic syndrome (MDS) in the treatment arm were more likely to achieve transfusion independence, but the difference vs placebo was not statistically significant.
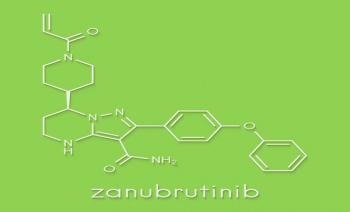
A post-hoc analysis showed that zanubrutinib resulted in fewer adverse events compared with ibrutinib.

Patient outcomes in this trial were compared between the regimens of lenalidomide, bortezomib, and dexamethasone and daratumumab, lenalidomide, bortezomib, and dexamethasone for use against multiple myeloma (MM).

Researchers collected feedback from 20 clinicians implementing a telehealth serious illness conversation (SIC) with their patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS).

In this new analysis, investigators review the late adverse events associated with anti-CD19 chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory B-cell non-Hodgkin lymphoma.

In the final part of our interview with Joseph Mikhael, MD, MEd, FRCPC, FACP, he addresses how patients who experience an early relapse of their multiple myeloma are predisposed to worse outcomes.

It is rare for patients with chronic lymphocytic leukemia (CLL) to present with ocular involvement, but a mutational test could help clinicians identify patients more quickly.

This meta-analysis study will estimate the global and regional burden of HIV-associated non-Hodgkin lymphoma to aid the development of prevention and control strategies.

In a recently published systematic review, researchers determined that VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome typically presents in men, with atypical morphology and with systemic symptoms.

In part 2 of our interview with Suzanne Lentzsch, MD, PhD, Columbia University's College of Physicians and Surgeon, she touches on potential new therapies, important clinical considerations, and treatment challenge for relapsed/refractory multiple myeloma.

Quality of life was also a top concern among patients when asked about their priorities if they experienced relapse or were refractory to therapy.
The 5 agents in ViPOR—including venetoclax and lenalidomide—worked better together in a phase 1b/2 trial than they previously have, individually, in treating patients with certain diffuse large B-cell lymphoma (DLBCL) subtypes.

In April, the FDA approved decabtagene vicleucel (ide-cel) for earlier treatment of relapsed/refractory multiple myleoma.

Study findings provide new tools to assess treatment preferences when using hypomethylating agents in myelodysplastic syndromes (MDS).

The FDA approved epcoritamab-bysp to treat adults with relapsed or refractory (R/R) follicular lymphoma after 2 or more lines of systemic therapy.

In the realm of lymphoma and chronic lymphocytic leukemia (CLL), and of the treatments that address them, a global survey of nearly 7000 patients proves there’s a long way to go to eliminate cancer-related fatigue from their lives.

People receiving hypomethylating agent (HMA) therapy spent 33 more days at home than people receiving anthracycline-based therapy in the first year after diagnosis.

Determining the roles that various inflammatory markers and pathways play in lower-risk myelodysplastic syndromes (MDS) could lead to therapies that keep disease progression at bay.
Researchers have found that specific genetic markers may increase the risk of cardiovascular adverse effects in patients using Bruton’s tyrosine kinase (BTK) inhibitors.












