
Hematology
Latest News
Latest Videos

CME Content
More News

The study highlights the importance of including patient self-assessment—and not just clinician assessment—when planning treatment in multiple myeloma.

In part 3 of a discussion with Andrew Kuykendall, MD, Moffitt Cancer Center, he talks of rusfertide’s ability to enable patients to live a more viable life and free them from being tethered to the need for regular phlebotomies.

Data from the ATLAS research comprises a series of trials that have yielded years of long-term, comprehensive data on the safety and efficacy of fitusiran (Qfitlia; Sanofi).

Erythropoiesis-stimulating agent (ESA) treatment before or early after regular transfusion therapy improved overall survival (OS) In lower-risk myelodysplastic syndromes (MDS), a new study found.

For higher-risk multiple myeloma (MM), successful patient selection and monitoring strategies are vital for the management of adverse events and the disease itself.

The American Society of Hematology (ASH) was already looking to expand its Bridge Grant program when changes to the National Institutes of Health (NIH) funding landscape created greater urgency.
Data incorporated for this study were collected from 9 centers in the UK focused on third-line and beyond chimeric antigen receptor (CAR) T-cell administration in patients with relapsed/refractory large B-cell lymphoma (LBCL).

A study of 200 patients with chronic lymphocytic leukemia (CLL) showed a lower rate of severe and serious adverse events among those treated with zanubrutinib compared with ibrutinib.
The rate of cGVHD-free survival was 78% at 1 year in patients who received Orca-T compared with 38% among patients who received standard allogeneic stem cell transplant for hematologic malignancies.

Treatment guidelines in polycythemia vera currently recommend maintaining hematocrit below 45%, with a higher threshold for men vs women.
Researchers also highlighted the significance of TTNT-D as a real-world measure of treatment efficacy, noting it provides insight into both clinical and patient-centered outcomes.

Cemacabtagene ansegedleucel, an allogeneic chimeric antigen receptor (CAR) T-cell therapy, is being investigated in relapsed/refractory large B-cell lymphoma.
Investigators found that lipid metabolism disruptions spark T-cell dysfunction in patients with chronic lymphocytic leukemia.

Fred Locke, MD, Moffitt Cancer Center, explains why this hematologic cancer is such an attractive target for chimeric antigen receptor (CAR) T-cell therapy, specifically allogeneic, which uses healthy donor cells.

Polycythemia vera is a classic myeloproliferative neoplasm and a chronic type of leukemia, which often leads to overproduction of various blood cells.
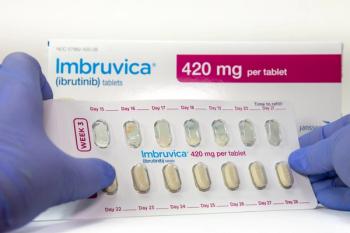
Ibrutinib remains a key treatment for relapsed chronic lymphocytic leukemia (CLL), but findings suggest that combining it with other therapies can improve response rates and may allow for treatment discontinuation in some patients.

Adverse physical functions were indicative of reduced survival and increased risk of immune effector cell–associated neurotoxicity syndrome (ICANS) in patients with non-Hodgkin lymphoma (NHL) previously treated with chimeric antigen receptor T-cell therapy.

A rituximab and lenalidomide combination for treating non-Hodgkin lymphoma (NHL) could play a greater role in future clinical trials.
Reducing intravenous (IV) isatuximab delivery from 75 minutes to 30 minutes could provide a wealth of benefits to patients with multiple myeloma (MM) and health care systems alike.

Interim data from the ECHELON-3 trial previously showed that adding brentuximab vedotin to lenalidomide and rituximab improved overall survival among patients who have relapsed or refractory large B-cell lymphoma (R/R LBCL).

Patients treated in community settings were more likely to be older, less likely to be White, and had worse survival rates, highlighting disparities in specialized cancer care.

Despite research showing the benefits of circulating tumor DNA as an indicator of disease remission in B-cell lymphoma, hurdles that include availability and cost remain to its widespread implementation.
The Connect MM Registry is the longest-running and largest registry in the US, providing invaluable prognostic insights into various patient and disease characteristics.

The CELESTIAL-301 trial evaluating SynKIR-310, a novel chimeric antigen receptor T-cell therapy, has dosed its first patient with relapsed/refractory B-cell non-Hodgkin lymphoma (B-NHL).
Extranodal non-Hodgkin lymphoma (NHL) makes up over one-third of NHL cases, yet remains understudied and in need of deeper research considerations.


















