
A recent study investigated what makes some non-small cell lung cancer patients with a type of human leukocyte antigen (HLA) called B44 are more likely than others to respond to immunotherapy.

A recent study investigated what makes some non-small cell lung cancer patients with a type of human leukocyte antigen (HLA) called B44 are more likely than others to respond to immunotherapy.

Researchers from the Children’s Medical Center Research Institute at the University of Texas Southwestern discovered a potential treatment for patients with non–small cell lung cancer (NSCLC) who have KRAS and LKB1 mutations.

Deferred low-dose computed tomography lung cancer screening during the coronavirus disease 2019 (COVID-19) pandemic has worsened outcomes for patients with lung cancer, a new study found.

Genentech's tiragolumab, a novel immunotherapy for non-small cell lung cancer with PD-L1 expression, is the first anti-TIGIT therapy to be granted Breakthrough Therapy Designation.

More Americans have received at least 1 dose of a coronavirus disease 2019 (COVID-19) vaccine than have tested positive for the virus; Biden administration funds mass production of a rapid over-the-counter COVID-19 test; breast cancer surpasses lung cancer as most common cancer worldwide.
Recently published results show that adding a limited course of chemotherapy to nivolumab plus ipilimumab in the first-line setting for non-small cell lung cancer is effective and tolerable.
Patients with both lung cancer and autoimmune disease have no significant difference in survival than patients with lung cancer alone, according to recent research from Northwestern University.
The 2021 American Cancer Society annual Cancer Statistics report found that the overall rate of cancer mortality continuously declined from 1991 to 2018 but also highlighted racial and geographic disparities.

There are several risk factors to review when determining whether or not a patient is high risk for lung cancer and should be screened, said Joanna Thompson, multidisciplinary program manager, Highlands Oncology Group.

This year’s top 5 most-read stories in lung cancer were overrun by non–small cell lung cancer drug trial results and FDA action.

FDA fact sheets on coronavirus disease 2019 (COVID-19) vaccines provide guidelines for special patient populations, including the immunocompromised.
NSCLC patients who developed immune-related adverse events during immunotherapy showed longer overall survival and progression-free survival than patients who did not.
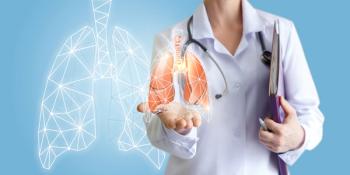
Women had higher survival rates after lung cancer compared with men, regardless of other risk factors, in a population-based registry study from the Karolinska Instituet in Sweden.

Highlands Oncology Group has found the most success getting the word out about its lung cancer screening program by directly engaging with primary care physicians (PCPs), said Joanna Thompson of Highlands Oncology Group.

Data from the Phase I CHRYSALIS trial also support an expanded access program that makes some patients eligible for amivantamab treatment while the FDA reviews the submission.
Physicians and patient advocates alike are working to spread the message that it is safe to continue on the course of cancer care and participate in clinical trials during COVID-19.

The Highlands Oncology Group partners with primary care providers to identify patients at high risk of lung cancer so the specialists can do a more in-depth screening, explained Joanna Thompson, multidisciplinary program manager, Highlands Oncology Group.
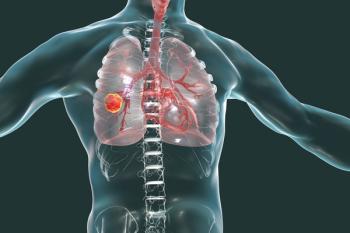
Early treatment with lorlatinib led to improved progression-free survival in ALK-positive NSCLC patients compared with crizotinib treatment, a study published in the New England Journal of Medicine found.

Multidisciplinary lung cancer clinics might help patients and the system save on treatment costs while improving patient experiences, according to an abstract presented at CHEST 2020.

In patients under age 70, lung cancer conferred the highest risk of death from coronavirus disease 2019 (COVID-19).
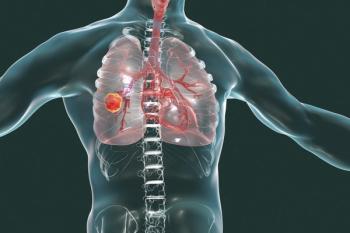
Current lung cancer screening guidelines do not identify high-risk young, African American smokers, an abstract presented at CHEST 2020 found.
Abstracts presented at CHEST 2020 explored possible reasons for low lung cancer screening rates and suggested solutions.
Regeneron’s PD-1 inhibitor Libtayo was accepted for priority FDA review for the treatment of first-line locally advanced or metastatic non–small cell lung cancer (NSCLC) with PD-L1 expression of at least 50%.
Current screening criteria in the United States identify fewer women than men who will get lung cancer, creating a perfect storm for what experts during a session at the CHEST Annual Meeting deemed an “invisible epidemic.”

A pair of abstracts based on a phase 1/2 trial of adagrasib (MRTX849) a KRAS G12C inhibitor, showed promising results for patients with lung, bowel, and other solid tumors, researchers reported at the 32nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics.

259 Prospect Plains Rd, Bldg H
Cranbury, NJ 08512
© 2025 MJH Life Sciences®
All rights reserved.
