Small Cell Lung Cancer
Latest News
Latest Videos

More News
Patients with small cell lung cancer (SCLC) with fewer than 5 brain metastases fared better than those with more than 5 when treated with stereotactic radiosurgery (SRS).
A retrospective case series study suggests that standardized guidelines for reporting and managing significant incidental findings (SIFs) in low-dose computed tomography (LDCT) lung cancer screening could help optimize the treatment of patients whose LDCT scans show SIFs.
Investigators found no significant impact on survival, however.
The majority of patients had prior experience with telemedicine, but none had experience with telerehabilitation.

Abstracts were presented in a session on metastatic lung cancer on the final day of the 2023 American Society of Clinical Oncology Annual Meeting.
A session chaired by ASCO and ESMO leadership included experts on the latest lung and colorectal cancer screening technologies and persistent disparities in screening access and uptake.
The phase 3 LUNAR trial evaluating tumor treating fields (TTFields) with standard-of-care therapies met its primary end point in patients with metastatic non–small cell lung cancer (NSCLC).

A study presented at the American Society of Clinical Oncology Annual Meeting found practice- and provider-level racial and ethnic inequities in rates of next-generation sequencing (NGS) testing for patients with advanced non–small cell lung cancer (NSCLC) treated in the community setting.
High circulating tumor DNA (ctDNA) tumor fraction after induction chemotherapy identifies a population with an unmet need who has low chances to benefit from immune checkpoint blockades for non–small cell lung cancer (NSCLC), according to research discussed during a presentation at the American Society of Clinical Oncology Annual Meeting.
An abstract at the American Society of Clinical Oncology Annual Meeting showed disparities in lung cancer screening rates in an urban, multiethnic community, and patient navigation increased rates overall.

Former and current smokers showed higher mortality from early-stage non-small cell lung cancer than never smokers.

Mark A. Socinski, MD, executive director at AdventHealth Cancer Institute, shares insight on the cost-effectiveness and utility of biomarker testing in non–small cell lung cancer (NSCLC).

Thomas Stricker, MD, PhD, and Gregory Vidal, MD, PhD, are both first authors on their respective abstracts involving patients with advanced non-small cell lung cancer in the community setting.

Researchers took both the learning curve of the new process and the COVID-19 pandemic into consideration.

Compared with first-line immunotherapy or chemotherapy alone, combination chemoimmunotherapy for advanced/metastatic non–small cell lung cancer has significantly higher antineoplastic drug and associated medical costs.
Osimertinib plus chemotherapy showed a statistically significant improvement in progression-free survival (PFS) vs osimertinib alone in patients with epidermal growth factor receptor–mutated (EGFRm) advanced non–small cell lung cancer (NSCLC)
Immune checkpoint inhibitors (ICIs) can increase life expectancy among patients who have non–small cell lung cancer (NSCLC), but they also carry the risk of immune-related adverse events.

Just 29% of patients listed a treatment-response goal among their top personal goals.

An analysis of the EMPOWER‐Lung 3 trial found that the addition of cemiplimab to platinum chemotherapy for non–small cell lung cancer (NSCLC) was associated with improved patient quality of life (QOL) vs chemotherapy alone.

Mark A. Socinski, MD, executive director of AdventHealth Cancer Institute, gave insight into treatment resistance and the importance of repeat biomarker testing at the time of disease progression in non–small cell lung cancer (NSCLC).

This cross-sectional observational study found several factors associated with whether a patient had sufficient lung cancer risk factor documentation in the electronic health record.

Mark A. Socinski, MD, executive director of the AdventHealth Cancer Institute, shares his insight on liquid- and tissue-based biopsies and their clinical utility in non–small cell lung cancer (NSCLC).
The American Society for Radiation Oncology (ASTRO) and European Society for Radiotherapy and Oncology (ESTRO) jointly released a clinical guideline on the use of definitive local therapy for the treatment of oligometastatic non–small cell lung cancer (NSCLC).
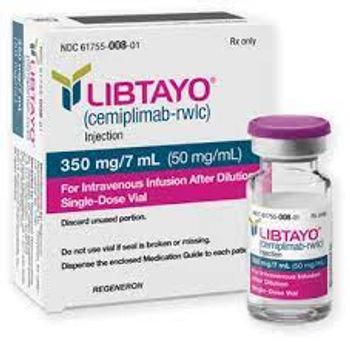
Final overall survival data were presented at the European Lung Cancer Congress in Copenhagen, Denmark.

Evidence-Based Oncology (EBO) spoke with Len Lichtenfeld, MD, the chief medical officer for Jasper Health and a board member with CancerCare.