Clinical
Latest News

Real-World Study Finds Brentuximab Vedotin With Chemo Is Safe, Effective in First-line Treatment of PTCL

In Data Race, Flatiron Health Touts Tripling of Global Oncology Research Network
Latest Videos

CME Content
More News
Switching to venetoclax led to sustained high rates of undetectable minimal residual disease, the investigators found.

Researchers found no significant differences in surgical outcomes or follow-up between housed and unhoused patients with osteoarthritis undergoing total joint arthroplasty, suggesting the procedure is safe and effective regardless of housing status.

Panelists discuss how newer immune-based therapies and bispecific antibodies may enable fixed-duration treatment approaches that could eliminate the need for stem cell transplant in older but fit patients, potentially allowing for treatment-free intervals after achieving deep responses.

Panelists discuss how to balance achieving deeper MRD-negative responses against increased toxicity risks in transplant-ineligible patients by personalizing therapy through dose modifications, weekly vs twice-weekly dosing schedules, and careful monitoring while maintaining treatment intensity similar to clinical trials.

Fewer than half of eligible patients with advanced ovarian cancer receive first-line maintenance treatments, highlighting care gaps.

Caroline Vovan, PharmD, CDE, emphasized the expanding role of ambulatory clinical pharmacists in value-based care.
A panelist discusses how ASCO 2025's most important breakthrough was the oral decitabine plus venetoclax combination, representing a potential paradigm shift if approved by the FDA, while highlighting that menin inhibitors (including the already-approved revumenib and pipeline agents like ziftomenib, bleximenib, and enzomenib) are the most exciting emerging therapies targeting 40% to 50% of patients with acute myeloid leukemia with NPM1 mutations or KMT2A rearrangements, though significant therapeutic gaps remain for challenging subgroups like TP53-mutated disease.

A panelist discusses how a large propensity score–matched analysis of 1300 patients aged 60-75 found similar all-cause mortality between intensive chemotherapy and azacitidine plus venetoclax, but with lower adverse events in the azacitidine-venetoclax group, suggesting that treatment selection should be individualized based on patient fitness, genetic mutations, transplant candidacy, and patient preferences for time-limited vs continuous therapy, while emphasizing the need for prospective randomized trials to definitively guide treatment decisions in this age group.

María Díez Campelo, MD, PhD, concludes by highlighting imetelstat’s real-world quality of life benefits for patients with lower-risk myelodysplastic syndromes (MDS) and the need for improved patient-reported outcome tools and caregiver-focused research.

Revumenib is now the sole targeted therapy recommended by the National Comprehensive Cancer Network for KMT2A-rearranged acute leukemia, which opens a path for payers, said Ivo Abraham, PhD, RN, of The University of Arizona.

Ambulatory clinical pharmacists improve patient outcomes and reduce health care costs by providing hands-on chronic disease management, patient education, and medication cost oversight, according to Caroline Vovan, PharmD, CDE.

Being overweight or obese may lower all-cause mortality risks in non–cystic fibrosis (CF) bronchiectasis patients, while being underweight increases health complications.

Glucagon-like peptide 1 receptor agonists may offer neuroprotective and survival benefits beyond blood sugar control.

ATLAS trial investigator Guy Young, MD, Children's Hospital Los Angeles and University of Southern California Keck School of Medicine, highlights the current treatment options for patients with hemophilia A or B, with or without inhibitors, since the FDA approval of fitusiran (Qfitlia; Sanofi).

In part 2, Hans Lee, MD, shares practical tips for using linvoseltamab in heavily pretreated multiple myeloma and outlines trials that may expand its future role.

Panelists discuss how reaching underserved populations requires proactive outreach, digital health tools with appropriate training, addressing health literacy barriers, and ensuring equitable access to diabetes technologies and treatments rather than waiting for patients to seek care.

Panelists discuss how clinical decision support tools, care pathways, and artificial intelligence can address primary care workforce shortages by providing real-time guidance, predictive modeling for high-risk patients, and autonomous agents for patient outreach and care coordination.

Caroline Vovan, PharmD, CDE, highlights how ambulatory clinical pharmacists contribute to value-based care by managing chronic conditions, improving medication adherence, and reducing costs.

Imetelstat (Rytelo; Geron) significantly improved and sustained health-related quality of life in patients with lower-risk myelodysplastic syndromes (MDS), with benefits linked directly to the drug rather than patient characteristics.
An expert discusses how different adverse event profiles of antibody-drug conjugates (ADCs) influence treatment decisions by requiring careful patient selection based on comorbidities like prior lung disease, implementing baseline assessments and monitoring protocols for pneumonitis and ocular toxicities, and recognizing that early detection and management of adverse effects allows for continued treatment through dose modifications.

An expert discusses how clinicians and institutions can streamline biomarker-driven therapy decisions by establishing rapid turnaround times for biopsies and pathology results, maintaining in-house testing capabilities, coordinating efficiently between interventional radiology and pathology teams, and ensuring insurance approvals don’t delay treatment initiation for patients who may deteriorate quickly.

The newly approved linvoseltamab (Lynozyfic; Regeneron) has a manageable safety profile, along with a patient-friendly dosing schedule that includes intravenous administration, step-up dosing, and de-escalation to monthly maintenance.

The phase 3 IMerge trial (NCT02598661) evaluated how treatment with imetelstat impacts health-related quality of life in patients with lower-risk myelodysplastic syndromes (MDS), according to María Díez Campelo, MD, PhD.
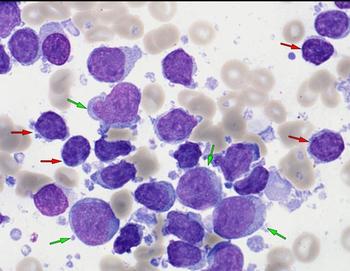
Real-world analysis reveals that undetectable CD20 expression worsens outcomes for patients treated with epcoritamab and glofitamab bispecific antibodies for their diffuse large B-cell lymphoma (DLBCL).

Three patient outcomes were measured in this new study: time to onset of ocular-related myasthenia gravis, Activities of Daily Living response, and Minimal Symptom Expression.