Clinical
Latest News
Latest Videos

CME Content
More News

A new review article highlights some of the newest therapies available to treat graft-versus-host disease (GVHD), but the authors say there is insufficient data to draw broad conclusions.

A pan-cancer reference atlas provides a framework for an immune-based patient stratification that study authors expect to be predictive for patient prognosis and immunotherapy response at an elevated level after further studies.

In patients refractory to conventional therapy, the combination resulted in a 70% reduction in a key biomarker compared with placebo.
Posters presented at AMCP Nexus 2021 evaluated the health care resource utilization (HRU) and costs associated with the chimeric antigen receptor T-cell therapies tisagenlecleucel and axicabtagene ciloleucel.
The report suggests factors besides molecular profiling can be helpful in patient stratification.

Two abstracts presented at the CHEST Annual Meeting 2021 showed that patients with narcolepsy treated with once-nightly sodium oxybate, FT218, vs placebo exhibited significant improvements in sleep latency and cataplexy.

Angelman syndrome affects roughly 1 in every 20,000 children and it has no approved treatment.
A report suggests that people with chronic obstructive pulmonary disease (COPD) and asthma have a protein in their lungs that leaks a small molecule into their bloodstream that restricts their breathing instead of relaxing their airways.
Mortality and liver-related complications increased with fibrosis stage in patients with nonalcoholic fatty liver disease (NAFLD).

Study findings suggest that the unique genomic variations of cerebrospinal fluid can be leveraged as a liquid biopsy to effectively and safely improve decision-making regarding treatment of patients with non–small cell lung cancer (NSCLC) with leptomeningeal metastasis.

Posters presented at the AMCP Nexus 2021 meeting reviewed the cost-effectiveness and cost per response for zanubrutinib vs ibrutinib.

A review of current molecular and electrophysiological biomarkers in spinal muscular atrophy (SMA) concluded that more exploration is necessary to find noninvasive, yet accurate, measures of disease progression and therapy response.
Autologous tumor lysate particle-loaded dendritic cell (TLPLDC) vaccination is well-tolerated in combination with other immunotherapies, but further research is needed to confirm its efficacy.

The benefit of real-world evidence is that it provides more data on subpopulations and diverse populations, said Christina Barrington, vice president of pharmacy programs at Priority Health.

A new study from Turkey shows, among other results, that delayed initiation of treatment with growth hormone among pediatric patients may lead to minimal height improvements despite adherence to the therapy.

Adalimumab biosimilar AVT02 exhibited comparative efficacy, safety, tolerability, and immunogenicity to the reference drug Humira in patients with moderate-to-severe chronic plaque psoriasis.
There are currently no FDA-approved treatments for refractory chronic cough or unexplained chronic cough. At the CHEST Annual Meeting 2021, attendees heard a readout from a durability study as well as pooled analyses from 2 phase 3 trials for gefapixant.

There has been a huge shift forward in the understanding of innate and adaptive immune mechanisms that provoke type 2 inflammation in atopic disease and eosinophilic esophagitis, according to a CHEST Annual Meeting 2021 speaker.

Multiple trastuzumab biosimilars were approved and launched very quickly in the United States, which sets up an interesting scenario in the market to see the nuance of having multiple options available, said Cate Lockhart, PhD, PharmD, MS, program, director, Biologics and Biosimilars Collective Intelligence Consortium (BBCIC).
At a session of the CHEST Annual Meeting 2021, specialists reviewed the need for a thorough diagnosis to tell whether a patient's symptoms are caused by asthma or another pulmonary condition.
Researchers at the CHEST Annual Meeting 2021 addressed the evolution of COVID-19 variants, how these emerging strains impact vaccines, and preventive recommendations for at-risk populations.

There are several reasons as to why precision therapeutics have not taken off for chronic obstructive pulmonary disease (COPD) in the same way that they have for other diseases, said Don Sin, MD, FRCP, MPH, a professor of respiratory medicine at the University of British Columbia and head of the Centre of Heart Lung Innovation, St. Paul’s Hospital.
Panelists of a session at CHEST 2021 discuss the latest research regarding efficacy and safety of therapies in the management of asthma, including biologics, corticosteroids, and more.
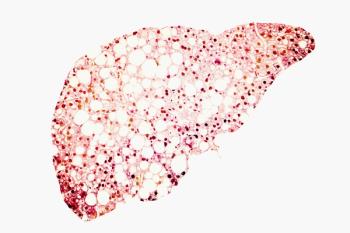
There was statistically significant and clinically meaningful overall survival benefit vs sorafenib as a first-line treatment for patients with unresectable hepatocellular carcinoma (HCC) in top-line results from a phase 3 trial.

Highlighting the latest ophthalmology-related news reported across MJH Life Sciences™.