
Experts debate e-cigarettes' role in smoking cessation and public health at ERS Congress 2025, weighing benefits against potential risks and regulatory needs.

Experts debate e-cigarettes' role in smoking cessation and public health at ERS Congress 2025, weighing benefits against potential risks and regulatory needs.

Integrating these care services can enhance patient QOL and address unmet needs in serious respiratory illnesses, explains Natasha Smallwood, BMedSci, MBBS, MSc, Monash University.

Experts reveal the health risks of indoor air pollution from biomass fuels and cleaning products, emphasizing prevention strategies for respiratory diseases.

Oncology stakeholders are navigating new policies as the landscape quickly evolves, according to Ryan Haumschild, PharmD, MS, MBA, CPEL.

Artificial intelligence (AI) holds the potential to democratize precision oncology, but it must be implemented thoughtfully.

In a time of skyrocketing oncology drug costs, the role of pharmacists in managing patients’ treatment and keeping care in the outpatient setting is more urgent than ever.

Teamwork between primary care providers and oncologists helps patients get needed care and achieve better results, according to Mark Fendrick, MD, at the Patient-Centered Oncology Care (PCOC) conference.

Kathy Oubre, MS, discusses opportunities for community cancer centers to expand service lines without sacrificing care quality.
Broader integration of artificial intelligence (AI) in precision oncology depends on overcoming barriers such as trust and transparency, according to Davey Daniel, MD.

Patients with hematologic malignancies face ongoing survivorship challenges as providers struggle to coordinate care, according to Brian Koffman, MDCM, DCFP, FCFP, DABFP, MSEd.

Panelists discussed the ingredients of successful handoffs at the time of cancer diagnosis and ways to collaborate as screening capabilities evolve.

Consolidation in oncology is creating access and cost challenges, whereas advocacy helps improve patient care and treatment availability, according to Sucharu "Chris" Prakash, MD.

Oncology utilization management often frustrates patients and payers, but collaboration and artificial intelligence can streamline processes and improve care quality, according to Vishnukamal Golla, MD, MPH.

As therapeutic advances enable patients with cancer to live longer, greater attention is needed to support the goal of survivorship, starting at diagnosis.

Explore cutting-edge discussions on patient-centered oncology, value-based care, AI innovations, and survivorship strategies Thursday and Friday at PCOC 2025 in Nashville.

Discover groundbreaking discussions at the European Respiratory Society Congress 2025, focusing on severe asthma, e-cigarette debates, and global health equity in respiratory care.

Patient-Centered Oncology Care (PCOC) conference co-chairs Kathy Oubre, MS, and Davey Daniel, MD, explain how the event unites experts to explore evolving oncology care and patient-centered strategies.

New findings reveal sotatercept's significant benefits for right ventricular function and tricuspid regurgitation in pulmonary arterial hypertension.

The combination of amivantamab and lazertinib in first-line non–small cell lung cancer (NSCLC) significantly reduces resistance mechanisms with implications for second-line treatment, said Danny Nguyen, MD, of City of Hope.

Remote patient monitoring enhances cancer care by improving outcomes and reducing emergency visits, despite challenges in reimbursement and technology access.

The subcutaneous dose had comparable efficacy to intravenous dosing while enhancing patient convenience in non–small cell lung cancer, explains Nicolas Girard, MD, PhD.

Community oncology leaders navigate challenges in value-based care under the Enhancing Oncology Model, facing performance payment uncertainties and evolving drug markets.
Updated results from the MARIPOSA trial show that amivantamab plus lazertinib significantly reduces EGFR- and MET-driven resistance compared with osimertinib.

Byoung Chul Cho, MD, PhD, of the Yonsei University College of Medicine, discussed outcomes in the COCOON trial of an enhanced dermatologic regimen with amivantamab-lazertinib treatment.

Subcutaneous amivantamab plus chemotherapy offers an equally effective treatment for EGFR exon 20 insertion non–small cell lung cancer (NSCLC), mirroring PAPILLON results of the intravenous formulation, explained Sun Lim Min, MD, PhD.
The FDA's removal of REMS for CAR T-cell therapies enhances access for community oncology, paving the way for innovative treatments and improved patient outcomes.

Panelists at the Community Oncology Alliance Payer Exchange Summit discuss the urgent need for innovative reimbursement models in cancer care to match advancements in biomedical technology and drug discovery.
Monthly SC amivantamab plus daily lazertinib demonstrated strong efficacy and tolerability in EGFR-mutated advanced NSCLC, providing a convenient alternative to IV dosing.

Personalized testing is preferred for patients with NSCLC with mutations, but there are challenges to implementing this testing, said Yang Xia, MD, PhD.
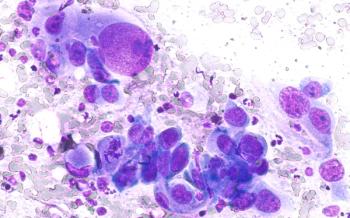
Central nervous system (CNS) metastases in EGFR-mutant non–small cell lung cancer (NSCLC) remain a major challenge, with emerging therapies and evolving trial designs aiming to improve outcomes and address unmet needs.